Science Topics – 124
In the cardiac myocytes, the strong inward rectifier K+ current (IK1 in the heart) determines the resting potential and facilitates the rapid repolarization at the final phase of action potentials. Because the open-channel conductance of the strong inward rectifier K+ current is apparently proportional to the "square root" of the extracellular K+ concentration ([K+]out), increases/decreases in [K+]out induce paradoxical increase/decrease in the outward currents of IK1 at membrane potentials near the reversal potential, which together with the shift in the voltage dependence of the current with the K+ equilibrium potential, affects the repolarization and action-potential duration of cardiac myocytes. In this study, we studied Kir2.1, which is the canonical member of the strong inward rectifier K+ channel, under the condition where currents showed little inward rectification by washout of cytoplasmic polyamines. The results showed that the external K+ is not required to open this channel and that the noted square-root proportionality of the open channel conductance to [K]out is mediated by the fast pore blockade by the external Na+, which is competitive with the external K+. The results reveal that the paradoxical increase/decrease in outward currents of cardiac IK1 during alternations in [K+]out, which accelerates/decelerates ventricular repolarization during hyperkalemia/hypokalemia, is caused by competition from impermeant extracellular Na+.
Ishihara K.
External K+ dependence of strong inward rectifier K+ channel conductance is caused not by K+ but by competitive pore blockade by external Na+.
Journal of General Physiology. 150(7): 977-989, 2018.
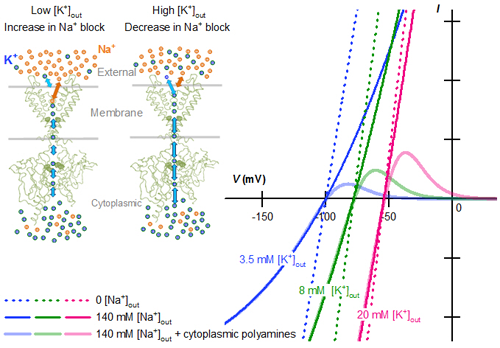
<Figure Legends>
External K+ concentration ([K+]out) dependence of strong inward rectifier K+ current reconstructed using the parameters of the Na+ block in competition with external K+.
Left, an increase in external K+ ions surmount the fast-pore block of the strong inward rectifier K+ channel by external Na+, thereby increasing both the inward and outward currents.
Right, theoretical current (I) – voltage (V) relationships calculated at 3.5 mM (blue), 8 mM (green), and 20 mM (magenta) [K+]out levels.
With no cytoplasmic polyamines, the I – V relationships are almost linear and the conductances show little [K+]out dependence in the absence of external Na+ (dotted lines with bold colors). As [K+]out is lowered in the presence of 140 mM external Na+, the conductances decrease due to the increase in the Na+ block (continuous lines with bold colors). In the I – V relationships showing a strong inward rectification due to the channel blockade by cytoplasmic polyamines, changes in [K+]out also alter the current amplitudes by enhancing/suppressing the Na+ block of polyamine-unblocked channels (continuous lines with faint colors).
Department of Physiology, Kurume University School of Medicine, Japan.
