Science Topics – 111
The hypothalamic-pituitary-adrenal (HPA) axis is activated by a variety of stressors, and corticotropin-releasing hormone (CRH), which is synthesized in the paraventricular nucleus (PVN) of the hypothalamus, plays a key role in HPA axis regulation. The cell bodies of CRH neurons are present in the PVN, from which their axons project to the median eminence (ME). CRH released from the ME activates the secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary, which subsequently activates the secretion of glucocorticoids from the adrenal glands. In non-stressful conditions, γ-aminobutyric acid (GABA)-ergic inputs exert an inhibitory action on CRH neurons in the PVN. This GABAergic inhibition requires relatively low intracellular Cl- concentrations ([Cl-]i), which are maintained by the outward-directing K+-Cl- cotransporter (KCC2). In contrast, if an animal is exposed to an acute stressor, GABA excites the CRH neurons as a result of an increase in [Cl-]i caused by KCC2 internalization.
In the present study, by using heterozygous GAD67-GFP knock-in mice which exhibited decreased GABA content, we found that the release of CRH was impaired, accumulating in the cell bodies of CRH neurons. The GABAA receptor (GABAAR) and the inward-directing Na+-K+-2Cl- cotransporter (NKCC1), but not KCC2, were expressed in the terminals of the CRH neurons at the ME. In contrast, CRH neuronal somata were enriched with KCC2 but not with NKCC1. Thus, [Cl-]i may be increased at the terminals of CRH neurons compared with concentrations in the cell body. Moreover, vesicular GABA transporter-containing GABAergic terminals projecting from the arcuate nucleus (ARC) identified by retrograde labeling, were present in close proximity to CRH-positive nerve terminals. Furthermore, in the CRH-GCaMP3 mice, a GABAAR agonist increased the [Ca2+]i levels in the CRH neuron terminals but decreased the [Ca2+]i levels in their somata. Additionally, the increases in [Ca2+]i were prevented by an NKCC1 inhibitor. Here, we propose a novel mechanism by which the excitatory action of GABA promote CRH release from axon terminals with NKCC1-driven high [Cl-]i in the ME (Fig).
In the ARC, several types of neurons, e.g., pro-opiomelanocortin (POMC) neurons, agouti-related protein (AgRP)/neuropeptide Y (NPY) neurons, and rat insulin promoter (RIP)-expressing neurons, monitor energy status and modulate behavioral and metabolic responses via insulin, leptin, ghrelin and glucose in the blood. Of these neurons, AgRP/NPY neurons and RIP neurons are both GABAergic. Interestingly, AgRP/NPY neurons express glucocorticoid receptors, so that this putative novel negative feedback loop might be involved in the modulation of HPA axis at ARC-ME level. Thus our findings here could be a novel modulator of HPA axis via hormonal monitoring in the ARC associated with such as feeding.
Keisuke Kakizawa, Miho Watanabe, Hiroki Mutoh, Yuta Okawa, Miho Yamashita, Yuchio Yanagawa, Keiichi Itoi, Takafumi Suda, Yutaka Oki, Atsuo Fukuda*: A novel GABA-mediated corticotropin-releasing hormone secretory mechanism in the median eminence. Science Advances 2: e1501723, 2016.
*corresponding author
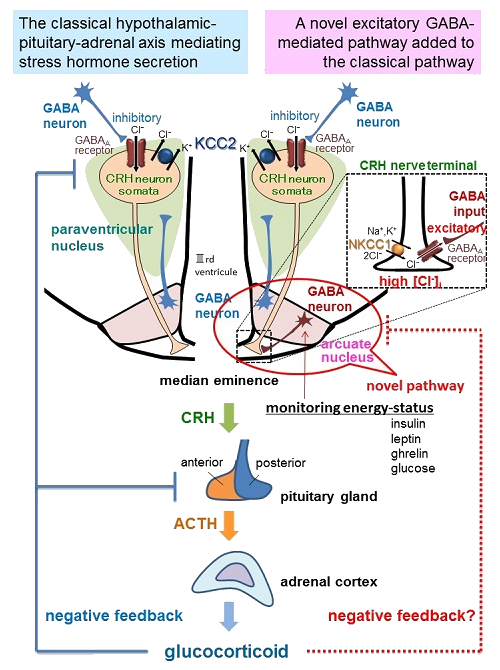
Fig. The classical hypothalamic-pituitary-adrenal (HPA) axis, and a novel excitatory GABAergic input from the ARC to ME promoting CRH release as a putative novel negative feedback loop monitoring energy status.
Department of Neurophysiology, Hamamatsu University School of Medicine, Japan
