Science Topics - 73
Cdk5 regulatory associated protein 1-like 1 (Cdkal1) has been associated with an impaired insulin response and increased risk of type 2 diabetes (T2D), but its molecular function has not been characterized. We have identified that Cdkal1 is a mammalian methylthiotransferase that specifically biosynthesizes 2-methylthio-N6-threonylcarbamoyladenosine (ms2t6A) at A37 of tRNALys(UUU) and that it is required for the accurate translation of AAA and AAG codons. To further investigate the physiological role of Cdkal1, we generated pancreatic b-cell-specific Cdkal1 knockout mice (Cdkal1 knockout mice). Cdkal1 knockout mice showed pancreatic islet hypertrophy, a decrease in insulin secretion and impaired blood glucose control. In Cdkal1-deficient -cells, misreading of Lys codon in proinsulin was observed, which results in decrease of proinsulin synthesis. Consequently, the pancreatic C-peptide level was significantly decreased in Cdkal1 knockout mice. Moreover, the expression of endoplasmic reticulum (ER) stress-related genes was upregulated in pancreatic b-cells, and abnormally structured ER was observed. Furthermore, Cdkal1 knockout mice rapidly developed severe glucose intolerance under high fat diet feeding condition. There was a global increase of ER stress response in b-cells of Cdkal1 knockout mice fed with a high fat diet. These findings suggest that the induced translation of proinsulin may require fully modified tRNALys(UUU), potentially explaining the molecular pathogenesis of T2D in patients carrying cdkal1 risk alleles. These findings have been published in the Journal of Clinical Investigation (doi:10.1172/JCI58056).
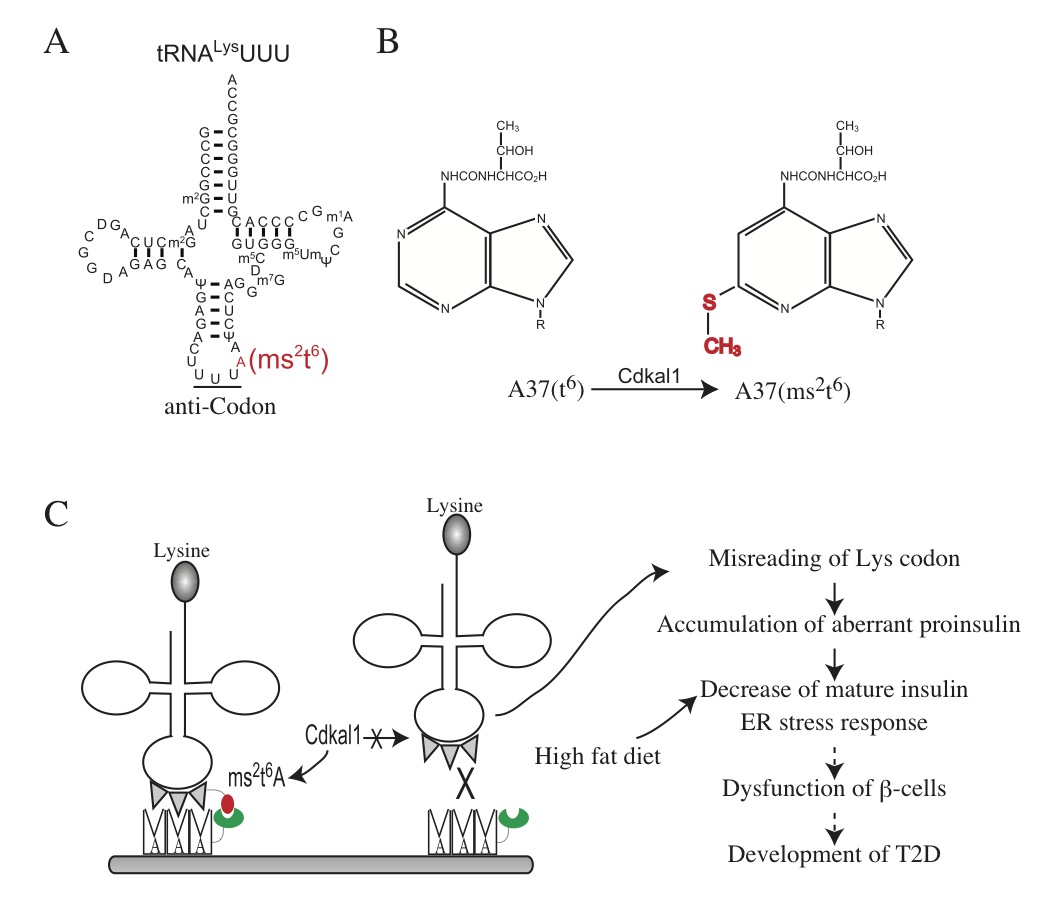
Figure Legend
(A) The secondary structure of tRNALys(UUU) is shown. (B) Cdkal1 catalyzes the conversion of N6-threonylcarbamoyladenosine (t6) to 2-methylthio-N6-threonylcarbamoyladenosine (ms2t6A) at A37 of tRNALys(UUU). (C) Proposed working model for regulation of b-cell function by Cdkal1 is shown.
* Department of Molecular Physiology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan
