Science Topics – 107
- Lab. of Integrative Physiology, Graduate School of Medicine, Osaka University
- Dept. of Physiology, Faculty of Medicine, Osaka Medical College
Voltage-sensing phosphatase, VSP, is composed of a voltage sensor and a cytoplasmic enzymatic domain. The enzymatic activity is evoked by the movement of the voltage sensor on membrane depolarization. Although a conformation change is essential for the enzymatic activities, little has been known about the conformation change not only of VSP but also of other proteins expressed on the plasma membrane because of technical limitations. In this study, we revealed conformation changes of the cytoplasmic enzymatic domain of VSP on the membrane using a newly developed technique, the genetic incorporation of a fluorescent unnatural amino acid, Anap. This technique brought several new findings. First, conformation changes were observed in some distinct part of the enzymatic domain within a few milliseconds after the voltage senor activation, meaning that the domain works as a single unit. Second, FRET experiment between the enzymatic domain and the plasma membrane showed that the enzymatic domain stays beneath the membrane irrespective of the voltage sensor activation. Third, the distinct fluorescence changes between the enzyme-active and –inactive type of VSP were found only when Anap was incorporated into the Cα2 loop in the enzymatic domain, suggesting that the Cα2 loop plays a role in the substrate metabolism. These results provide a new view that the enzymatic domain of VSP changes conformations to bind to the substrate without changing the distance toward the membrane and then it metabolizes the substrates accompanied by the Cα2 loop operation.
Voltage-dependent motion of the catalytic region of voltage-sensing phosphatase monitored by a fluorescent amino acid.
Sakata S*, Jinno Y, Kawanabe A and Okamura Y*. Proc Natl Acad Sci USA (2016) in press. *Corresponding authors
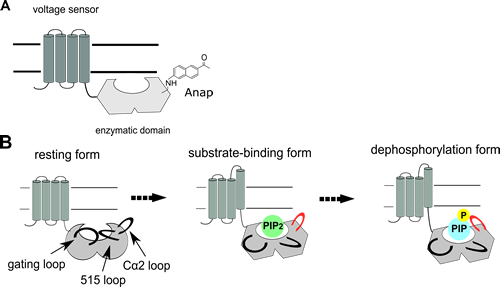
Figure A. A fluorescent amino acid, Anap, was incorporated into VSP on the plasma membrane.
Figure B. Conformation change associated with the enzymatic activity.
The voltage sensor operation evokes conformation changes of the enzymatic domain, which results in the binding of the substrate on the active center of the enzyme (substrate-binding form). And then, the enzymatic domain metabolizes the substrate accompanied by the Cα2 loop operation (dephosphorylation form).
